I had the opportunity to do some field work for an extended period of time this summer. Logistics of field work, travel, insurance, and other field related prep work aside, I also had a large number of plants that needed to be taken care of. I had to make plans for said plants.
What ended up happening was roping in a few people to check in on my plants during my absence, and distributing them so no one person was saddled with watering 50 + plants with very particular needs. Instructions were, water when the soil looks and feels dry, approximately once every 10 days or so for the larger plants, and once a week for the smaller ones.
Had I been a little more prepared and had more time to plan, here are the things I would have done. The following information has been compiled from my past experience with leaving plants with roommates for just under 2 weeks, and typically being entirely absent during the holiday season. The good news is, we can test out these ideas soon! I’ve put off this post for so long that it aligns with my departure for 2 weeks, perfect to test out some of my ideas and rescue any plants that did not fare well in between. Its worth noting that there are some complicating factors that I didn’t account for, such as the change in seasonality, living in a drier environment than before, use of artificial lighting, plantlets recently released from their humid propagation boxes, blooms on the way, etc. And I might have pests. Woohoo.
Tl;dr
- get plants in semi-hydroponics used to a higher water reservoir as to maximize fill before departure
- stick plants in soil over large jar of water (without touching) as to provide moisture
- minimize light sources (reduce plant growth/activity/water uptake)
- keep cuttings in water vessels with narrow mouths
- cluster similar plants together
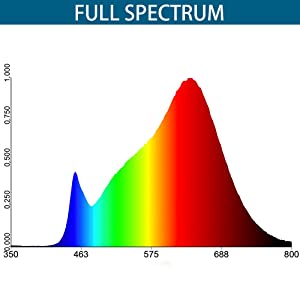
Let’s talk about why I came up with these points. Plants require a few things to live (and grow). Water, light (also heat), and nutrients. Of these, water and light (Fig. 1) are the critical ones for indoor plants when it is not the growing season. Additionally, the balance between water and light is very important and ideally mimics the plant’s native environment or initial growing conditions. Nutrients are not considered for shorter term absences.
Bottom humidity – fancy bottom watering
Watering without being present sounds like an easy thing to do. Just stick a bulb in or set up a drip system. This might work for some plants, but in my case, I predominantly have aroids and semi-succulents. These plants typically want to be watered in cycles of being fully soaked and then subsequently dried out until the next watering cycle. Nonetheless, most of my plants enjoy more moisture than the internet leads me to believe and typically live in a slightly more moisture rich environment in lieu of full desiccation. Thus, to maintain access to water without the ability to drench it from time to time (typically around 1 week’s time) can be a little difficult.
My solution is to stick all of my small pots over clean Classico mason jars after I use up all the sauce. The jars are filled about half way, and I pop the small nursery pots into the jar lid a couple of days after watering, so it maintains some moisture and will not fully dry out due to the nearly enclosed environment. This only works for my plants in pots of 2.5” diameter or smaller. For larger plants, self-watering pots are also an option, though I have typically found they are very finicky and require some calibration to prevent oversaturation of the base soil layer.
Hydroponics
Alternatively, I can provide endless amounts of water instead. This would be putting my plants into water directly, with or without additional media, but definitely not with soil. This method requires far more time to set up, but it is certainly doable. There are a few benefits of doing this. A deep water reservoir, precise control over nutrients, minimal pests… The trick is that it doesn’t work for every plant and it takes some time for plants previously living in soil to switch into water. Some I have cuttings fully growing out in just water, and some I have supported in a soil-less medium that wicks up water with strong root growth (this also adds aeration, very important to prevent a hypoxic environment!). The biggest complication with this method is cost – time and dollars. The second is that not all plants survive the transition, and it requires careful maintenance during that time.
For larger plants, transitioning into semi-hydro and filling up the jars as much as possible is ideal. If that is not an option, then the plant will simply not be watered during my absence. Plants in large containers tend to have more established root systems and reservoirs for instances of drought. Tropicals will not love this, but succulents will do just fine if they are large enough.
No sun or little sun
For the plants where I can’t set up water access via a large pool beneath the pot, I remove the water entirely. This leads to the plant missing a key ingredient for growth. In which case, perhaps the plant should not grow at all.
If there is no light, then there is no strong reason for water uptake. Most of my plants are suitable for “bright, indirect” sunlight. This is a cryptic statement that many a plant owner struggles with. What it tends to translate to is, most of my plants prefer as much sunlight as I can give them, so long as I bump them slowly into the sun. On the flip side, limiting sunlight will limit and slow growth. Less growth also means less water uptake and might set it off on a hibernation mode. Which is perfect if you can’t water your plants.
Grouping
Why group plants together? For context, my groupings are roughly: semi-succulent, semi-epiphytic, problem plants, and one-offs. These groupings are by water/light requirement, and care. The semi-succulents will need next to no care while I am gone. The semi-epiphytic plants and aroids would like some care. The problem plants need to be regularly checked on (these have pests, and I am not opposed to having them die-off entirely). And the one-offs are those that need to be watered, or left alone entirely. This just makes positioning my plants and making sure none get left out if there is someone checking in on them easier.
Survival of the fittest
Naturally, the plants might still be unhappy or die off while I’m gone. So what survives, survives. That’s a useful indicator for future trips what kinds of plants will do okay. In the chance I have to leave for an extended period of time again, then I’m all set with what did alright last time. Alternatively, if I really want plants around, cheap and robust plants are the way to go.
3+ weeks of travel
Things that actually happened during the field season: I split up some plants to pass along to others to take care of. At least one was on death’s door (it shriveled up, and the other similarly sad looking plant now has 1.5 leaves). I had a pet pass away during this time as well, I have no real thoughts on how to improve this situation for extended periods away other than living with someone else that is equally bonded to the animals. The rest of the plants are doing alright, though upon return, they did acquire some unwanted critters that were slowly killing them off.
Post field season thoughts: So that’s plants. What about the person travelling? I’ve been left with some thoughts after the last round of field work, and having just returned from a conference last week. One. Showers are nice. Two. I should start setting up a “recovery” station for myself when I return, especially if there is a big change in climate. Three. I want to find some time for myself to mull over what it means to be attached to various possessions, and how that affects me when I am absent or when I return after an extended period.
NEW: 2 week trial
Prepping for 2 weeks away, I’d like to avoid throwing my plants into hibernation. Stopping the growth of the young plants would simply lead to their death, or a tricky care situation on my return. Just about all of my smaller plants are in hydroponics or still suitable for a humid container. These will be under timed grow lights to compensate for the loss of sunlight with the change of seasons. The larger plants not in a reservoir will be going entirely unwatered, with two exceptions that are newer to my grouping of plants. Two smaller plants will also need to be watered during my absence, one is in the middle of slowly putting out flowers and requires the dry/wet cycle, and the other is entirely epiphytic in nature and does not possess functional roots. I have a few rooted cuttings, some will be staying in water, and some are being transitioned to semi-hydro and need to be topped off until the roots extend further. I was vaguely aware of needing to prepare them for my departure if I wanted healthy plants about 10 days pre-departure. During those 10 days, I purchased multiple grow lights and automatic timers, pulled out all the plants from propagation (except for one experimental box), informed my brother how to take care of my carnivorous plant (keep it flooded with rain water), and scattered hopefully live nematodes over all my potentially thrippy plants. I do still have some plants on campus, where they will be watered once during my absence. I may have written an entire spreadsheet with watering instructions and tips.
Expectations: All plants will be alive, and either slightly underwatered or overwatered when I get back. My calathea ornate (has spider mites currently and previously died down to a singular leaf) will likely go back to having one leaf. The corm only state oxalis may return from their hibernation and start putting out new leaves. The anthurium forgetii might be crispy. The propagations will not have made significant progress rooting. I will lose the hoya blooms on my linearis. If there are to be any casualties, I expect them to be amongst the newly propagated and the hoya I have had for weeks that have shown zero signs of growth (I think they had root rot before they even made it to my hands). Lastly, I might have aphids. I really hope this is not the case, but I have been side eyeing some of the leaf damage on my anthurium, and it is not looking promising.
Setup:
- X2 barrina T5s – “full spectrum”
- X1 barrina T5 – “full spectrum”
- X1 sansi 15 W bulb – “full spectrum”
- X1 barrina T5 (not shown)
- X2 GE automatic timers with two outlets set to turn on from 7:30 am to 10:30 pm ET (not shown)
- X3 misc coolermaster laptop fans (not shown)
- 4L of rainwater (not shown)
I purchased noma bulbs (8 W) sometime last year and also purchased a clamp lamp holder for it. I’ve been using it for nearly a year as a regular light bulb as well, and it has generally been focused on my carnivorous plants. I purchased the sansi bulb as an experiment when I moved home, with the intent of hanging them via pendant lamp bulb holders (since returned due to overheating). It now lives in the clamp lamp. All noma bulbs are currently inactive. They provided the lowest intensity light and had a tendency to heat up more than I would have liked. Once I find holders that are better at dissipating heat, they will likely make it back into regular use. I later purchased a set of 8 barrina T5s (yellow).
In the meantime, I have a vertical plant stand next to a window (Fig. 2). The plants on the stand above the window sill receive no additional artificial lighting other than those under the sansi bulb. There is one plant at a lower level that has heavy leaves, so it’s on the ground in case it topples. That one receives an off axis barrina light since it does not need high light intensity. These two are linked to one timer.
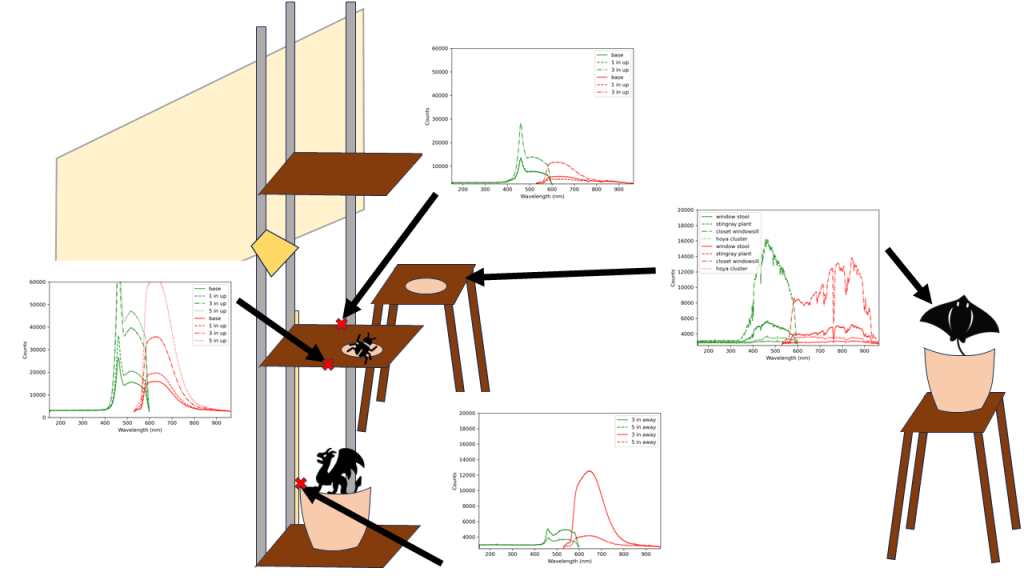
There are two barrinas linked together in the “closet” area, with the lights generally facing downwards about 5 – 20 inches away from the plant tops (Fig. 3). Another one is linked to the same timer and is focused on my previously “bathroom” plants (not shown in images). There are three fans in the closet, since I spotted some fluffy mold growing in the three days I had been gone. Ew. That’s probably what I get for no airflow and using rain water that has been sitting in a bucket of decaying leaves.
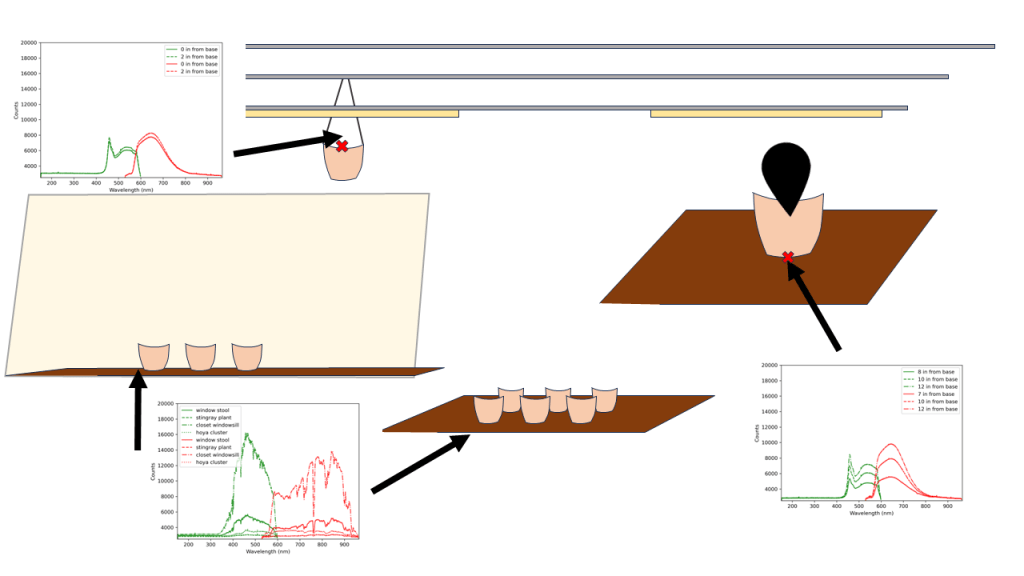
Various other plants are scattered about, in the hopes that natural lighting and overflow from growlights will be sufficient. To get a baseline, I decided to borrow – with permission! – a couple of spectrometers from the lab covering the UV and NIR range, and a cosine corrector. Samples were taken between 2:40 pm to 3:15 pm ET at 100 ms per sample and averaging over 10 samples. What I should be expecting to see is a curve similar to that provided by the barrina T5 listing (Fig. 1).
It looks like the Sansi bulb is doing a lot of heavy lifting, even on the far side of the stand. The “full spectrum”-ness seems to be covered reasonably well, though it certainly is not a smooth slope as otherwise shown in the images. The “oneoffs” show the light coming in from a window on a cloudy day. Both the stingray alocasia and hoya cluster are quite far from the window (~2m and 1m respectively), but the hoya cluster may additionally be receiving some light from the barrinas in the closet. That said, you can see the fall off from the barrina lights if they are not angled correctly from the dragonscale alocasia readings. The window stool may also be receiving some light from the sansi bulb, and is located about 40 cm away from the window sill. Even at this distance, the light intensity falls off quite a bit in comparison to spectra taken at the window sill.
The spectra looks as it should, based on the marketing images for the barrina lights. Though it is worth pointing out the apparent fall off around the 570 nm wavelength may be a function of the spectrometer rather than a lack of light in that wavelength. We can say that the sansi light bulb did a better job of outputting in the relevant spectrum than the natural light right by the window sill. It also does not have the red wavelength “peak”, and is more focused towards the blue light. Natural light coming in from the windows falls off very quickly, but the results might be different had it been a sunny day outside.
Conclusion: TBA